Virginia Tech Carilion Research Institute scientists Deborah Kelly and Sarah McDonald are ambitious and determined, and it’s paying off.
The two assistant professors recently received a National Institutes of Health grant for their collaborative work developing new imaging technologies that will allow them to see live rotavirus activity.
McDonald and Kelly, whose offices share a wall and whose laboratories are adjacent, began collaborating more than two years ago.
“I was interested in examining RNA-related processes using high-resolution imaging technology,” Kelly said. “Dr. McDonald’s work with rotavirus provided the right opportunity to collaboratively develop innovative tools together, using the virus as a model system.”
Kelly focuses on developing new imaging platforms – specifically those that help capture the dynamic structure of functionally important proteins in human cells. Traditionally, scientists use cryo-electron microscopy to image protein complexes.
With the capability to capture more details than ordinary light microscopy, electron microscopes can be used to peer at the invisible world around us, revealing unique information about macromolecules that can affect human health. The samples are frozen in place, keeping the structures close to how they would appear naturally. Researchers take snapshots of the structures and use computational algorithms to construct 3-D models of the imaged structures.
The problem with the cryogenic process is that it also freezes functions. Other scientists have achieved high-resolution images of immobile samples, including rotavirus, but they’ve yet to capture high-resolution action shots of real-time events.
That’s where McDonald enters. She studies rotavirus, which causes more than half a million deaths in infants and children every year, primarily in the developing world.
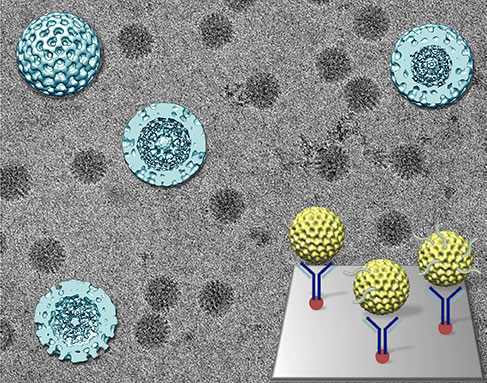
Rotaviruses have three layers, like a foil-wrapped chocolate egg. The innermost layer, the sweet cream, contains double-stranded RNA, the genetic material needed to create more viruses, and polymerases. Polymerases are responsible for manufacturing the messenger RNA molecules that infect a host.
When the virus is active, it sheds the outer layer – the foil. Scientists believe that shedding activates the polymerases. The chocolate layer is already permeated with holes, letting the RNA snake out. The virus in this form is called an active double-layered particle.
Inactive double-layered rotavirus particles were previously imaged at a moderate resolution in the 1990s, producing 3-D models that give information about how the active form of rotavirus operates on a molecular level. With Kelly’s improved technology, McDonald and Kelly say they think they can determine even more details about how the virus functions and infects host cells.
“The first aim of our grant is to use cryo-electron microscopy to image actively transcribing double-layered particles in a greater level of detail than is currently known,” McDonald said.
This higher resolution may lead to a better understanding of the functions of rotavirus.
“For the past two decades, the only available 3-D structures of actively transcribing double-layered particles have been at lower resolution,” McDonald said. “These active particle structures show messenger RNA exiting the double-layered particles, but they don’t reveal any detailed features inside the particles.”
When McDonald and Kelly took preliminary images, they discovered something interesting. The virus either appears as a well-organized golf ball or as a blurry blob. It was thought that the golf ball version was an active virus – that the organized appearance reflected a busy interior.
What McDonald and Kelly found, however, is that the opposite is true. The organized and calm exterior actually reflects a dormant interior, while the murky version of the viral particle indicates activity.
“For many years scientists have been concerned with higher-resolution results and haven’t paid close attention to the subtle diversity that exists in virus samples,” McDonald said. “But that diversity may be indicative of how viruses actually function inside cells. They’re not static, but dynamic in nature.”
To address that dynamic nature, McDonald and Kelly want to go beyond static snapshots of frozen rotavirus double-layered particles. They have another aim for their grant: liquid imaging.
In these new imaging applications, the samples are encased in a dynamic, fluid environment. This process creates an almost natural environment for the samples, allowing them to still function. The aim is to visualize more complicated processes, like the actively transcribing rotavirus particles.
With the grant, Kelly will continue to improve the imaging technologies while McDonald will continue to amass knowledge about how the rotavirus functions. Ultimately the two say they hope to be able to observe the virus as it occurs, without any manipulation that could influence its behavior.
“This National Institutes of Health grant will help Drs. Kelly and McDonald advance their research,” said Michael Friedlander, executive director of the Virginia Tech Carilion Research Institute. “The grant not only reveals the importance of this line of research, as recognized by leaders in structural biology and molecular virology, but it also reflects the synergy of their innovative collaboration.”
– Ashley Wenners Herron.